In organic chemistry, a functional group is a group of atoms or bonds inside a material that is responsible for the substance’s distinctive chemical reactions. Chemical reactions that are similar or identical will be carried out by two molecules of different sizes but with the same functional groups. The existence of a functional group in a molecule indicates that the molecule’s behaviour and chemical reactions may be anticipated in a systematic manner.
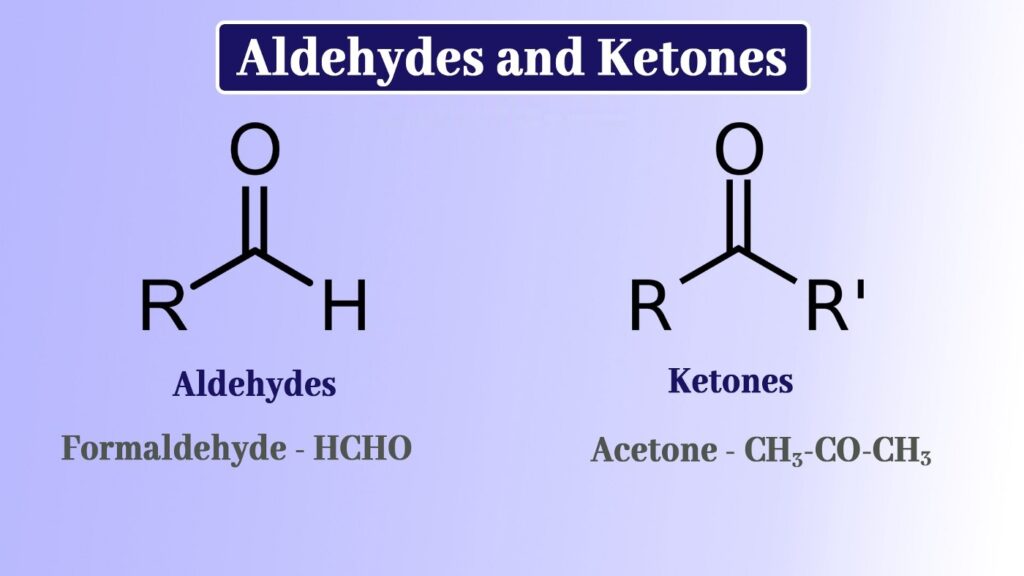
The carbonyl functional group, C=O, is present in aldehydes and ketones. These are organic compounds having the structures -CHO and RC(=O)R’, respectively, where R and R’ are carbon-containing substituents. Acetal is formed when aldehyde and ketones combine with alcohol in an acidic environment. In this article, we will discuss more on the aldehyde, ketones and their reactions.
Aldehydes
Aldehydes have one hydrogen atom connected to the carbonyl group, as well as a hydrogen group or a second hydrogen atom, which can be the one carrying a benzene ring or an alkyl group. The structure of aldehydes is an sp2 hybridised core carbon with a double bond to oxygen and a single bond to hydrogen.
- Small aldehydes are the ones that are most water-soluble.
- Acetaldehyde and formaldehyde are excellent examples. These two aldehydes are also quite essential in the industrial world.
- Aldehydes, in general, either polymerise or oligomerise.
Ketones
The carbonyl group in ketones has two hydrocarbon groups connected to it. These might be those with benzene rings or those with alkyl groups. A ketone’s carbonyl group does not have a hydrogen atom attached to it.
- Because of the existence of a polar carbonyl group, ketones are always polar. As a result, they have greater boiling points than non-polar substances.
- Since no hydrogen is connected to an oxygen atom, it cannot produce alcohols with any intermolecular hydrogen connection.
- As pi electrons shift in ketones, they have larger dipole moments than ethers or alcohols.
Aldehyde and Ketone Occurrence
Aldehydes and ketones are abundant in nature when combined with the other functional group. Plants and microorganisms include chemicals such as vanillin (vanilla bean), Citra (lemongrass), cinnamaldehyde (cinnamon bark), helminthosporal (a fungus toxin), camphor (camphor trees), and carvone (spearmint and caraway). Human and animal-derived chemicals include testosterone (male sex hormone), progesterone (female sex hormone), cortisone (adrenal hormone), and muscone (musk deer).
Reactions of Aldehydes and Ketones
Aldehydes and ketones undergo a series of reactions to produce a variety of compounds. Nucleophilic addition reactions are the most prevalent, leading to the creation of alcohols, alkenes, and diols, among other things.
Due to the steric and electrical factors, aldehydes are frequently more reactive toward nucleophilic replacements than ketones. A small hydrogen atom is connected to one side of the carbonyl group in aldehydes, while a bigger R group is attached to the other. R groups, on the other hand, are connected to both sides of the carbonyl group in ketones. Aldehydes, on the other hand, exhibit less steric hindrance than ketones.
Addition of Water
The production of a hydrate occurs when water is added to an aldehyde. This reaction is catalysed by small quantities of acids and bases. This happens because acid induces protonation of the carbonyl group’s oxygen, resulting in the development of a complete positive charge on the carbonyl carbon, making it a suitable nucleus. The nucleophile is changed from water (a weak nucleophile) to a hydroxide ion when hydroxyl ions are added (a strong nucleophile). Ketones do not normally produce stable hydrates.
Addition of Alcohol
The procedure begins with carbonyl oxygen protonation, which makes the C=O bond extremely electrophilic, which is subsequently attacked by alcohol, forming the oxonium intermediate. The aldehydes react with alcohols to form hemiacetal (a functional group made up of one —OH group and one —OR group connected to the same carbon) or acetals (a functional group made up of two —OR groups bound to the same carbon). The two reactants are combined to make the hemiacetal. An acetal is generated when the two reactants are mixed with hydrochloric acid.
Summary
Ketones and aldehydes are two simple chemical molecules with a carbonyl group. A double bond between carbon and oxygen exists in a carbonyl group. Because the carbon atom in the carbonyl group lacks reactive groups like Cl or OH, aldehydes and ketones are relatively simple organic molecules.